Posts Tagged ‘medical writing’
ICH E6 (R3): Implications of Recent Updates for Medical Writers
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) recently released a third revision of its E6 Guideline, also known as ICH E6 (R3). This revision introduces new concepts and updates in the realm of clinical trial methodologies, with an emphasis on quality, flexibility, and modernization. Let’s explore the key updates and discuss how they might affect medical writers.
Read MoreMy Fellowship in Medical Writing: What I Learned
By Katelyn Rivas Last year I had the pleasure of participating in the Synterex Regulatory Writing and Medical Communications Fellowship. I worked with and learned from Synterex staff members across all areas of the business, including medical writing, project management, quality control (QC), publishing, and business operations. Now that I have completed my fellowship and…
Read MoreHey ChatGPT? Tell Me a Story
We asked ChatGPT to tell us a story about a medical writer; here’s what it got right and what it got wrong. Like many of you, when ChatGPT appeared in the news recently (mainly in reference to stories about students misusing the technology), I was curious. There have been many times in recent years when…
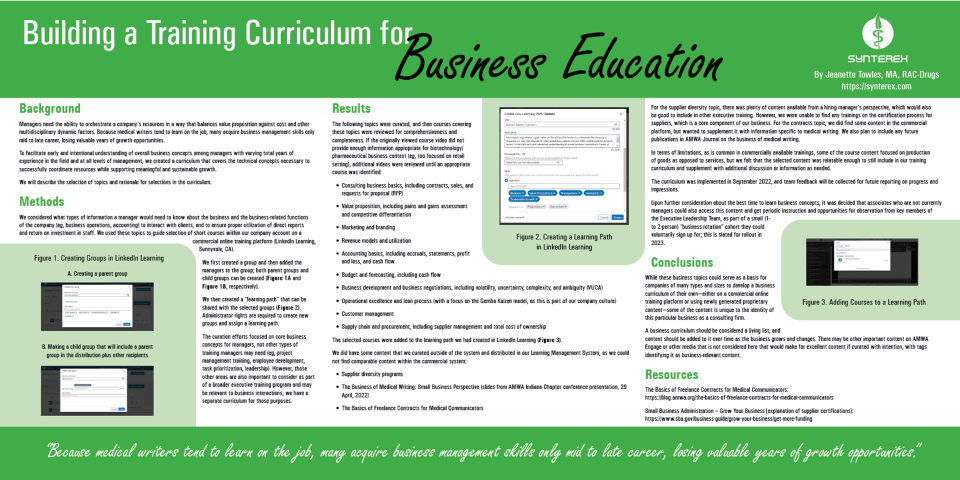
Read MoreBuilding a Training Curriculum for Business Education
Business skills matter. Download this poster, presented by Synterex at the 2022 American Medical Writers Association (AMWA) conference, or scroll down for a text-only version. Background Managers need the ability to orchestrate a company’s resources in a way that balances value proposition against cost and other multidisciplinary dynamic factors. Because medical writers tend to learn…
Read MoreDealing with Difficult People
Difficult people exist in any profession. Download this poster, presented by Synterex at the 2022 American Medical Writers Association (AMWA) conference, or scroll down for a text-only version. Sooner or later, you’ll have to work with someone whose personality clashes with yours. They may be a coworker, a client, a vendor, a boss, or a…
Read MoreChecklists for Medical Writers
Checklists are a great tool. Download this poster, presented by Synterex at the 2022 American Medical Writers Association (AMWA) conference, or scroll down for a text-only version. We have a complicated job. Every day there are new tasks to manage, learn, and get right. No matter how much experience and training you have, relying on…
Read MoreKey Takeaways for the Medical Writer from FDA’s Public Meeting: Supporting the Future of Rare Disease Product Development
FDA’s public meeting on February 24th left no uncertainty as to the future direction of drug development in rare disease: Patient-centric approaches that incorporate patient and caregiver input at all levels to ensure that clinical trial endpoints are robust and meaningful; and Utilization of patient data (registries, cases, natural history, and real world evidence) to…
Read MoreDefining ‘Medical Writer’ in a Job Description When the Role Defies Definition
When you look at job descriptions for medical writers, have you noticed that, regardless of the size of the hiring company, you end up with an array of interpretations of the role? Here are some examples: Medical writers may or may not write briefing books, write or review labels, or assist in regulatory responses Medical…
Read More